Pharmaceutical GMP – Complete Guide
The pharmaceutical industry has faced a number of challenges in recent years. They must rely on Good Manufacturing Practices (GMP) to reduce the costs associated with non-quality, to maintain their competitiveness, to reduce the risks associated with the sector and to better protect consumers.
To ensure that your sterile product manufacturing processes comply with pharmaceutical GMP, call on EREA.
Customized Technical Solution
Static and Dynamic protection
Quick installation and Implementation
Easy Surface Cleaning
Would you like an estimate for your project?
Do not hesitate to contact us for a personalized quote.
Introduction to Pharmaceutical Good Manufacturing Practice (GMP)
According to the WHO, pharmaceutical GMP is defined as one of the elements of quality assurance. These regulatory standards guarantee that the manufacture and control of products comply with the quality standards in force. These standards are announced in the marketing authorisation.
The French pharmaceutical GMPs date back to 1978. They aim to manage two types of risk:
- Risks of cross-contamination of products.
- Risks of confusion, particularly with regard to labelling and component identification.
GMPs apply to all aspects of the pharmaceutical manufacturing process: validation of manufacturing steps, storage, transport, laboratory facilities, traceability of files, instructions and operating procedures, etc.
On 22 August 2022, a new version of GMP Annex 1 was published. This will come into force in August 2023. It introduces a number of changes, such as strengthening the Pharmaceutical Quality System (PQS), introducing an approach based on Quality Risk Management (QRM) and making it compulsory to implement a Contamination Control Strategy (CCS).
Pharmaceutical Good Manufacturing Practices (GMP)
Pharmaceutical GMP imposes a certain number of requirements to ensure that the drug manufacturing process takes place under optimum conditions and to reduce the risk of contamination. Pharmaceutical manufacturers must comply with these standards to meet the various challenges they face. By complying with GMP, they can protect their reputation by guaranteeing the quality and safety of their products. Batch recalls and negative consumer reports are also significantly reduced. GMPs also aim to protect patients from non-quality products.
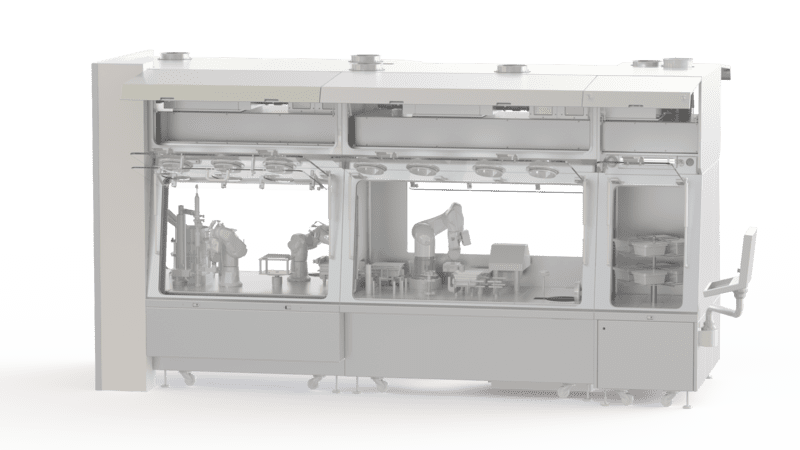
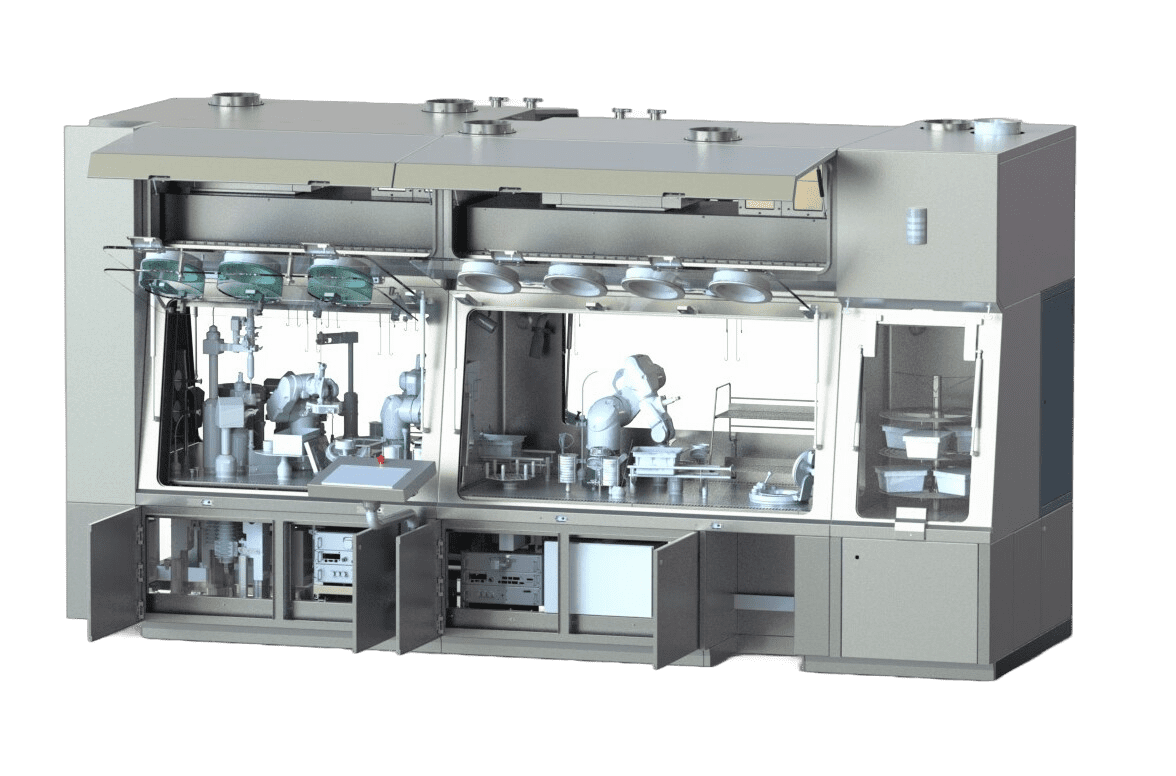
Good Manufacturing Practices take account of technological developments. They accept new containment solutions for pharmaceutical manufacturing processes: isolators or restricted access barrier systems (RABS). These new devices have the advantage of being efficient and economical. They are essential for ensuring that each stage of the manufacturing process is carried out in a contamination-free environment: mixing, weighing, filling, encapsulation, primary packaging, secondary packaging and so on. Cross-contamination can be physical, chemical or biological in origin.
The manufacturing process must comply with the requirements defined by GMP. These apply not only to the production of medicinal products, but also to the working environment, equipment, training and compliance with hygiene rules. Each stage of manufacture must be documented to facilitate control and monitoring.
Implementing Pharmaceutical GMP in Industry with EREA Pharma
Implementing pharmaceutical GMP is never easy. For better support, call on EREA. We master the regulatory standards in force, including GMP. We can advise you on the right solutions to adopt to effectively protect your pharmaceutical products during manufacture and to ensure they comply with regulations. Our team takes your needs into account before proposing tailor-made solutions. EREA is a designer and manufacturer of isolators, restricted access barrier systems (RABS) and various other containment solutions for the pharmaceutical industry.
For more information, don’t hesitate to contact our team of experts, who will get back to you as soon as possible.
Our Isotechnical Products
Feasible adaptations, reliable sterile processes and tests, safe and fast handling: Erea has 30 years of experience at your service.
Do you have a question? Do you have a specific need? Don't hesitate to contact our technicians
Don't hesitate to contact us for personalised information about our isotechnical products.