
by EREA Pharma | Oct 10, 2025 | Company, Event
Another day at the A3P Association Biarritz. Come along to our stand R29 for a chat over a coffee!

by EREA Pharma | Oct 2, 2025 | Company, Event
Try your luck and win a Kobo Elipsa 2E tablet! For our participation at A3P Biarritz this October, we’re launching a fun photo challenge that’s sure to make you smile! How to join this cheerful adventure? It’s as easy as 1-2-3: Stop by our booth R29 on Tuesday 7th and...
by EREA Pharma | Sep 25, 2025 | Company, Event
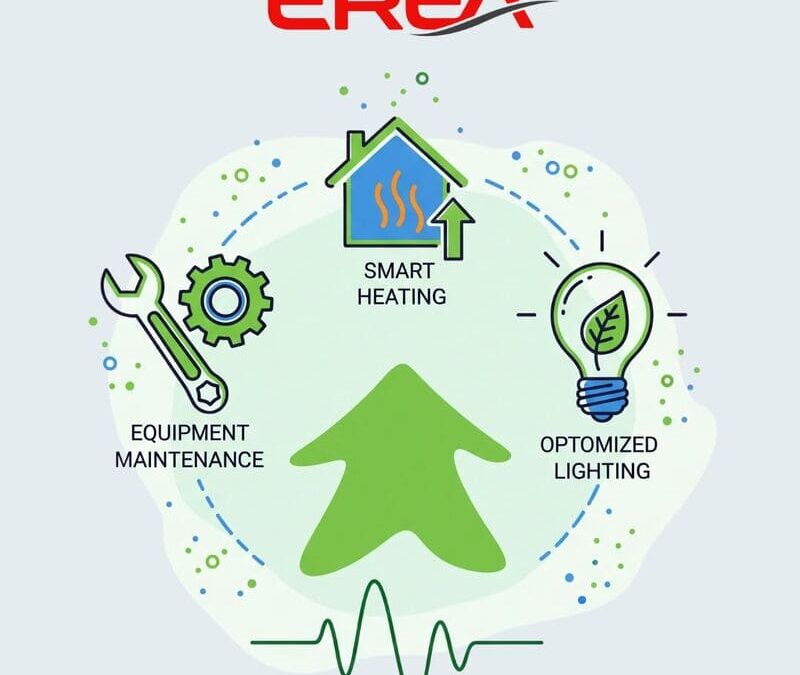
At EREA, our business growth goes hand in hand with a strong commitment to managing our energy consumption. Following a comprehensive energy assessment, we’ve set a clear goal: to reduce our consumption while maintaining a high-quality work environment for our...

by EREA Pharma | Sep 23, 2025 | Application, Company
We’re proud to announce the successful completion of a project involving a biodecontamination airlock! Both the FAT (Factory Acceptance Test) and SAT (Site Acceptance Test) were successfully validated, marking key milestones in our collaboration with the client....

by EREA Pharma | Sep 2, 2025 | Company, Event
The sun rises at EREA! After a well-deserved break for most EREA employees, we’re all full of energy and good humor! We’re ready to get back to your projects and continue to support you in your most ambitious innovations and challenges. Don’t...

by EREA Pharma | Aug 1, 2025 | Company, Event
Summer is here, and it’s time for some of our team to enjoy a well-deserved rest. Over the next few weeks, our LinkedIn communication will also be taking some time off. We’re taking a short break, time for everyone to recharge their batteries. We’ll...






