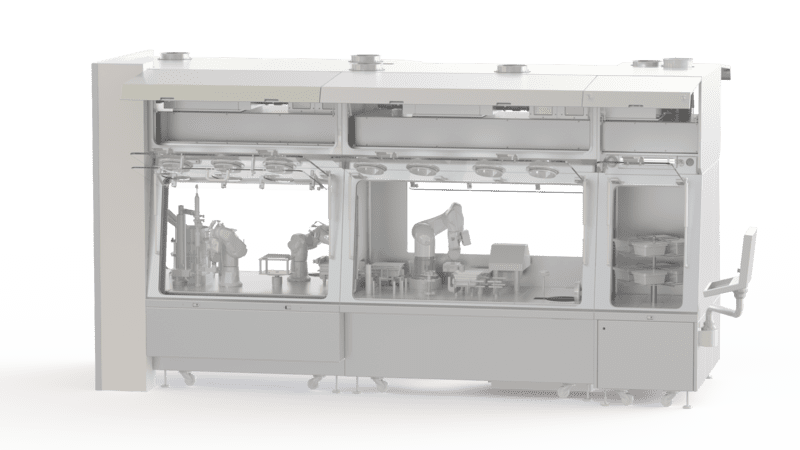
Process Analytical Technology (PAT) in Biopharmaceutical Manufacturing

What is Process Analytical Technology (PAT)?
Process Analytical Technology (PAT) is a systematic approach used in biopharmaceutical manufacturing to design, analyze, and control production processes through real-time measurements of critical parameters.
This technology aims to ensure product quality while improving manufacturing efficiency.
Customized Technical Solution
Static and Dynamic protection
Quick installation and Implementation
Easy Surface Cleaning
Would you like an estimate for your project?
Do not hesitate to contact us for a personalized quote.
Importance of PAT in Biopharmaceutical Manufacturing
In biopharmaceuticals, PAT is crucial for ensuring product consistency and quality. It allows real-time process monitoring, reducing error risks and production costs. Additionally, it fosters process optimization and continuous innovation.
Main Tools and Techniques of PAT
Spectroscopy
Spectroscopy is used to analyze the chemical composition of samples in real-time. It detects variations in raw materials and intermediates, ensuring consistent and high-quality production.
Chromatography
Chromatography separates and identifies components in complex mixtures. In biopharmaceutical manufacturing, it monitors protein purification and other biomolecules.
Data Analysis and Modeling
Advanced data analysis and modeling tools interpret the vast amounts of data generated by PAT. These tools help predict process performance and identify critical control parameters.
Benefits of Implementing PAT
Improved Product Quality
PAT enables continuous and precise process monitoring, ensuring that finished products meet the highest quality standards. This reduces quality variations and ensures patient safety.
Reduced Production Costs
By detecting process deviations early, PAT reduces waste and rework, lowering overall production costs. It also optimizes resource use and improves process efficiency.
Increased Innovation and Flexibility
Integrating PAT into biopharmaceutical manufacturing promotes innovation by allowing rapid and flexible process adjustments. It also facilitates adaptation to new technologies and changing regulatory requirements.
Process Analytical Technology (PAT) is an essential component of modern biopharmaceutical manufacturing. By providing advanced means of process control and optimization, it ensures the production of high-quality drugs efficiently and economically. The adoption of PAT will continue to transform the biopharmaceutical industry, bringing significant benefits to manufacturers and patients.
Our Isotechnical Products
Feasible adaptations, reliable sterile processes and tests, safe and fast handling: Erea has 30 years of experience at your service.
Do you have a question? Do you have a specific need? Don't hesitate to contact our technicians
Don't hesitate to contact us for personalised information about our isotechnical products.