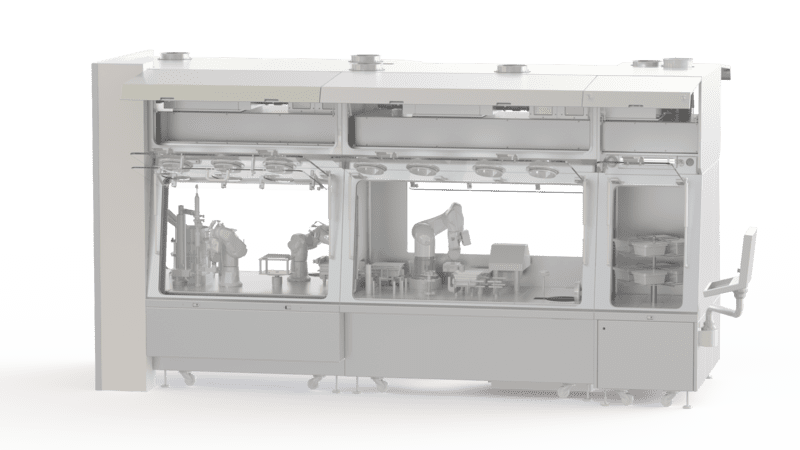
RABS for Pharmaceutical Industry
Introduced in the 1990’s, the Restricted Access Barrier System (RABS) is a barrier technology that offers effective product protection by providing a high level of separation between operators and the critical aseptic zone.
EREA offers solutions adapted to the needs and requirements of the pharmaceutical industry with a range of RABS guaranteeing protection of the product, the operators and the environment. You can choose between open RABS (active or passive) and closed RABS.
Customized Technical Solution
Static and Dynamic protection
Quick installation and Implementation
Easy Surface Cleaning
Would you like an estimate for your project?
Do not hesitate to contact us for a personalized quote.
Strict compliance with Annex 1 of GMP 2022-2023: How the update of Annex 5 impacts RABS applications in the pharmaceutical industry
The restricted access barrier system is one of the solutions proposed by Good Manufacturing Practice or GMP to guarantee the quality of medicines. This is a regulation governing the manufacture of pharmaceutical products. A new version was drawn up in 2022 and has been in force since August 2023. This is the revised Annex 1. It is more demanding in terms of compliance with sterility conditions in order to ensure product quality, avoid over-frequent product recalls and better protect consumers.
Annex 1 of the FPG introduces a number of changes. It highlights quality risk management (QRM) and contamination control strategy (CCS). Pharmaceutical companies must take these two points into account when carrying out their various processes. If RABS for the pharmaceutical industry is validated by the revised GMP Annex 1, it is because it also wishes to integrate new technological advances into the improvement of the manufacturing process for sterile products.
Evolution of RABS applications to meet the requirements of Annex 1 of GMP 2022-2023
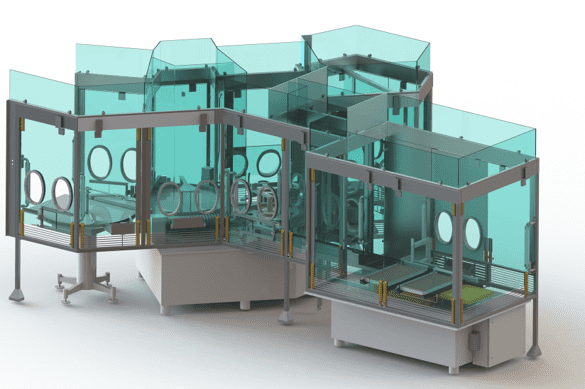
RABS is an innovative solution for carrying out pharmaceutical processes under sterile conditions. It has been designed to replace the old cleanroom, which is more expensive, less efficient and more difficult to maintain. The restricted access barrier system is the evolution of the cleanroom. It offers a better level of sterility assurance (SIA). It has the advantage of being more efficient thanks to the fixed, rigid barrier it is equipped with. It is also more economical and easier to maintain.
Successful integration of RABS at EREA Pharma for state-of-the-art aseptic pharmaceutical manufacturing.
FAQ about Pharmaceutical RABS
What is the difference between a pharmaceutical RABS and an isolator?
RABS and isolators are two pieces of equipment widely used in the pharmaceutical industry. Although there are similarities between the two, they are not identical. RABS for the pharmaceutical industry is a barrier technology. A fixed, rigid separation is placed between the operator area and the critical process area. However, this separation is not strict and the environment remains identical to a conventional room. Isolators, on the other hand, create a controlled aseptic containment space in which the critical stages of the process take place with maximum safety. They incorporate a decontamination system with a sporicidal agent (H2O2) that guarantees decontamination of all internal surfaces, further controlling the risk of contamination.
What should I choose,a pharmaceutical RABS or an isolator?
There are a number of parameters to consider when choosing between a pharmaceutical RABS and an isolator. In addition to regulatory requirements, you also need to take into account product toxicity, system flexibility, overall installation and running costs, production start-up time, energy consumption and operator training time.
What is the difference between active and passive flow pharmaceutical RABS?
A passive-flow pharmaceutical RABS has no dedicated air system. Instead, the air flow in the enclosure comes from the outside via integrated fan and filter systems. The equipment is said to be active if it has an air ventilation system that is independent of the production room airflow.
How do I get a pharmaceutical RABS?
The best way to install a restricted access barrier system is to contact a designer and manufacturer of containment solutions, such as EREA. They can provide you with a tailor-made pharmaceutical RABS that takes account of your specific requirements.
Our Isotechnical Products
Feasible adaptations, reliable sterile processes and tests, safe and fast handling: Erea has 30 years of experience at your service.
Do you have a question? Do you have a specific need? Don't hesitate to contact our technicians
Don't hesitate to contact us for personalised information about our isotechnical products.